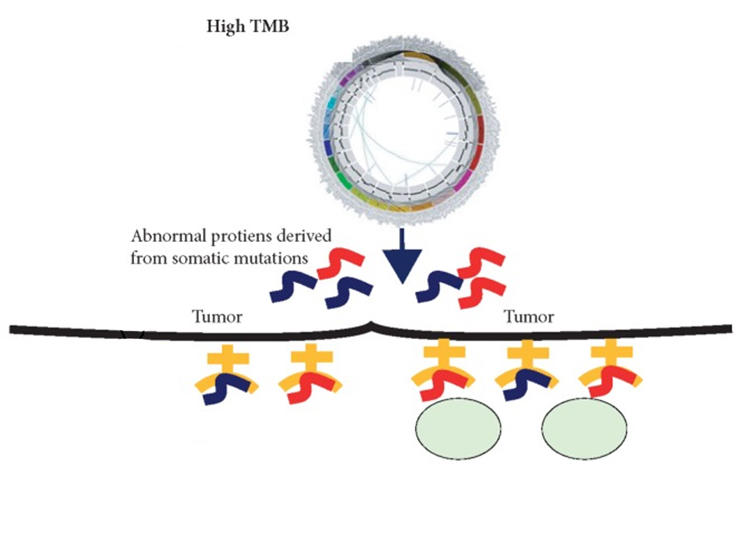
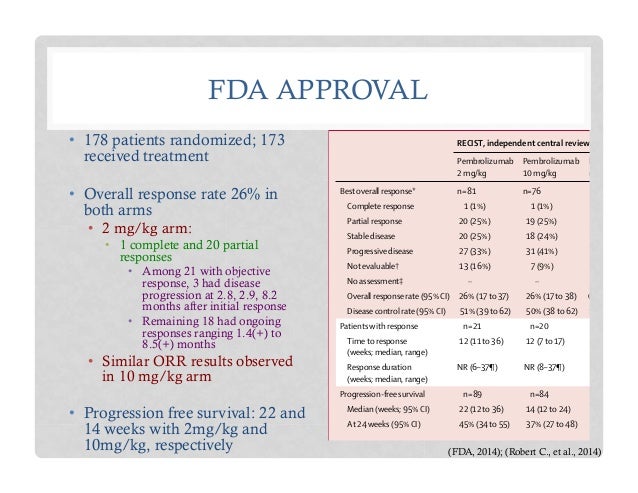
![PDF] FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors | Semantic Scholar PDF] FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/d87ced43628213babff1c81f9a45fcf02a22d940/2-Figure1-1.png)
PDF] FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors | Semantic Scholar

FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors | Clinical Cancer Research

FDA Approves Updated Indication for Merck's KEYTRUDA® (pembrolizumab) for Treatment of Certain Patients With Urothelial Carcinoma (Bladder Cancer) | Business Wire
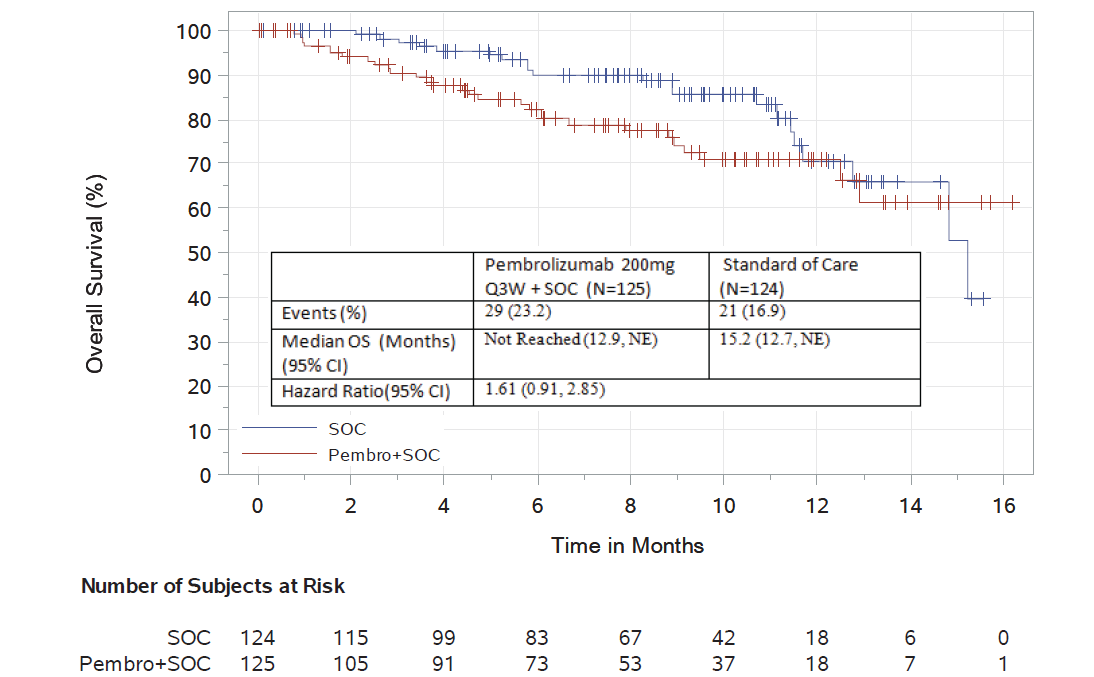
FDA Alerts Healthcare Professionals and Oncology Clinical Investigators about Two Clinical Trials on Hold Evaluating KEYTRUDA® (pembrolizumab) in Patients with Multiple Myeloma | FDA

FDA Grants Accelerated Approval to Pembrolizumab in Combination with Pemetrexed and Carboplatin for Treatment of Metastatic Non-Squamous NSCLC | ONS Voice

A reality check of the accelerated approval of immune-checkpoint inhibitors | Nature Reviews Clinical Oncology

Pembrolizumab KEYNOTE-001: an adaptive study leading to accelerated approval for two indications and a companion diagnostic - Annals of Oncology